
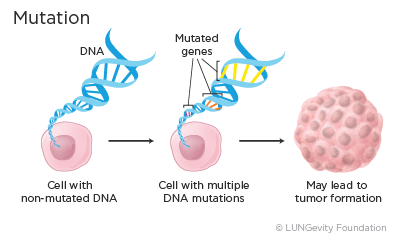
All the organs and tissues in our bodies are made up of cells, and each of these cells contains thousands of genes. Genes are made up of DNA, which is a specific code that is used to ultimately make proteins that have specific functions in cells. It is essential for each gene to have the correct DNA code, or instructions, for making its protein. When the DNA is correct, the protein is able to perform the correct function.
When a gene has an error in its DNA, it is said to be mutated. Mutations occur often, and normally the body can correct them. However, depending on where in a gene the mutation occurred, the mutation may become part of the cell’s blueprint. Over time an accumulation of mutations can result in the formation of a tumor. Mutations that can cause cancer are called driver mutations.
Mutations can be:
· Acqured (also called somatic): Present only in the tumor and not passed on to childrren
· Inherited (also called germline): Present in all cells of the body and passed on to children
Virtually all of the mutations that occur and inform treatment decisions in lung cancer are acquired. Inherited mutations are still being researched in lung cancer.
All of the targeted therapies discussed here are for acquired mutations.
What Are the Different Types of Driver Mutations That Are Known to Cause Cancer?
Several types of driver mutations cause cancer. Some of these include:
· Activating mutation: An activating mutation is a change in the DNA sequence that can cause changes in the protein made by the gene so that it is always active. This may lead to uncontrolled cell growth.
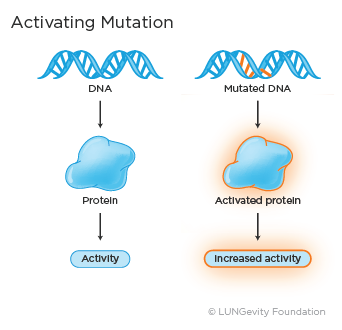
Examples of activating mutations in lung adenocarcinoma are an L858R substitution mutation or exon 19 deletion in the epidermal growth factor receptor (EGFR) gene and the V600E mutation in the BRAF gene.
· Fusion: Fusion, or rearrangment, occurs when a part of one gene fuses with, or attaches to, a part of another gene. The fused gene then produces a unique protein that promotes abnormal, uncontrolled cell growth.
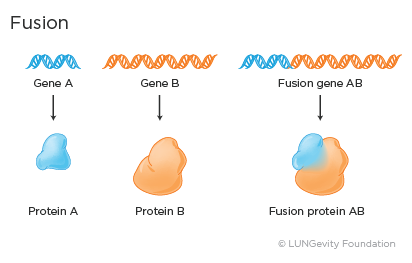
Examples of fusion genes in lung adenocarcinoma include the ALK-EML4 and the CD74-ROS1 fusion genes.,
· Amplification: Amplification means that there are many more copies of a gene than normal. The over then leads to increased protein activity and uncontrolled cell growth.
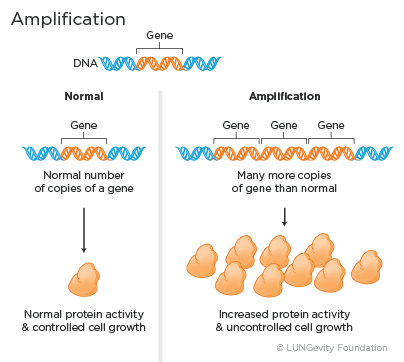
Examples of genes that can be amplified in lung adenocarcinoma include the HER2 and the MET genes.
· Deletion: Deletion means part of or the entire gene is missing in the cancer cells. The deletion then leads to reduced levels of protein being produced by the cancer cell.
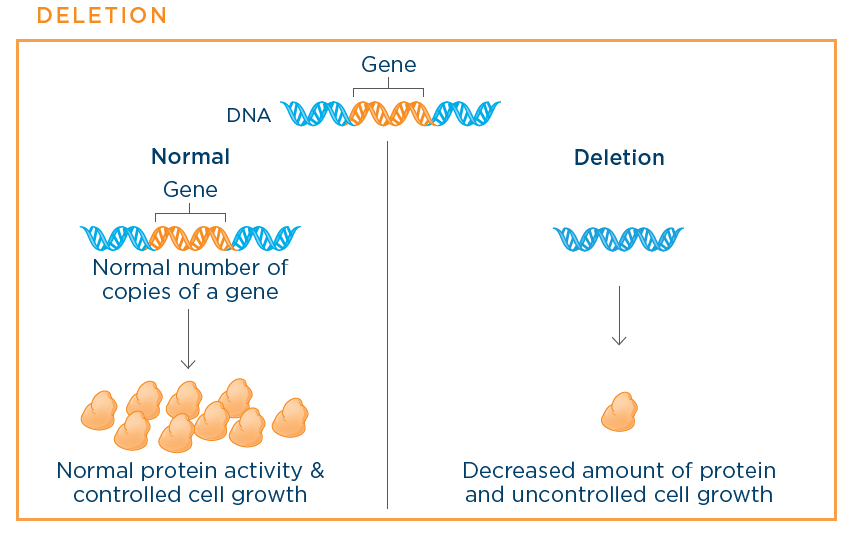
Examples of deleted genes in small cell lung cancer (SCLC) include the TP53 and the RB genes.
Lung cancer describes many different types of cancer that start in the lung or related structures. There are two different ways of describing what kind of lung cancer a person has:
A person’s lung cancer may or may not have one of the many known driver mutations. So far, scientists have identified more than 20 different driver mutations that can be found in non-small cell lung cancer (NSCLC) and small cell lung cancer, and they are continuing to look for more.
These driver mutations are biomarkers that can be identified through molecular (or genomic) testing of a lung cancer. This testing is typically performed on a piece of tumor taken from a biopsy or, in some cases, through a blood test or liquid biopsy. (Read below for more information about liquid biopsies.) Their presence may determine whether a patient will be prescribed one of the targeted therapies approved by the US Food and Drug Administration (FDA) or be potentially eligible for a clinical trial.
Right now scientists have the most information about driver mutations in the histologic subtype of lung cancer called adenocarcinoma.,
The driver mutations that currently have targeted therapies approved by the FDA include ALK, EGFR, ROS1, and BRAF V600E.

Researchers are also making progress in understanding mutations in squamous cell lung cancer, although there are no FDA-approved drugs yet for these.,,,10

Driver mutations in small cell lung cancer and other types of lung cancer are also being studied. However, there are as yet no targeted therapy drugs that are FDA-approved for them. This may change, so check with your doctors.11
Targeted therapies are sometimes also called:
Targeted therapies are a type of treatment that targets specific parts of cancer cells and the signals that proteins send to cancer cells that cause them to grow and divide uncontrollably.12 These drugs are often grouped by how they work or what part of the cell they target.
All of the targeted therapy drugs that have aleady been FDA-approved for lung cancer belong to a class of drugs called tyrosine kinase inhibitors (TKIs).
Tyrosine kinases are specific proteins that act as enzymes that may signal cancer cells to grow. The proteins encoded by the ALK, EGFR, ROS1, and BRAF genes are all examples of tyosine kinases. Tyrosine kinase inhibitors are targeted therapies that block these cell signals.13 By blocking the signals, they keep the cancer from growing and spreading. TKIs are named based on the enzyme, or protein, that they block. The driver mutations for which there are FDA-approved drugs on the market are:
In addition, clinical trials are currently studying promising drugs to target other driver mutations.
An anaplastic lymphoma kinase (ALK) rearrangement is a fusion between two genes: ALK and, most commonly, echinoderm microtubule-associated protein-like 4 (EML4). (Note that the ALK gene can rarely be fused to other genes.) The fusion of these two genes produces an abnormal ALK protein that causes cancer cells to grow and spread.
About 7% of patients with lung adenocarcinoma in the US have tumors with an ALK mutation. A similar frequency has been reported in Asian populations. The fusion between ALK and EML4 is more common among younger patients (median age at diagnosis is 52 years), nonsmokers or light smokers, and those with adenocarcinomas. It has rarely been found in patients with squamous cell carcinoma., ,14
There are currently four FDA-approved ALK inhibitors:
In addition, other ALK inhibitors are currently being studied in clinical trials.
ALK inhibitors work by blocking the signals that the abnormal ALK proteins send to cells to grow and divide uncontrollably. This stops the growth and spread of the cancer cells.19
? HOW are aLK inhibitors ADMINISTERED?
The side effects of the ALK inhibitors differ by drug and by patient.15,16,17,18
Some common side effects of ALK inhibitors as a group include:
Some of these side effects can be improved by reducing the dose of ALK inhibitors.15,16,17,18,20
Some serious but rare side effects of ALK inhibitors as a group include:
Overall, these drugs are well tolerated.
In addition, crizotinib (Xalkori?) has unique vision-specific side effects. These include:15
Low testosterone is one source of fatigue in patients being treated with crizotinib (Xalkori?). This can also lead to sexual dysfunction and depression. Researchers have found that hormone replacement therapy is an effective method of managing these side effects.21
When you start a new ALK inhibitor, you should discuss with your doctor:
Get tips on managing treatment-related side effects.
Sometimes treatment with an ALK inhibitor will be the only treatment a patient receives. However, in most cases, ALK inhibitors are used before, together with, or after other treatments, which can include chemotherapy, surgery, and/or radiation therapy.
Epidermal growth factor receptor (EGFR) is a protein found in abnormally high levels on the surface of some cancer cells. Driver mutations involving EGFR can lead to uncontrolled cancer cell growth and survival.19
Approximately 10% of patients with NSCLC in the US and 35% in East Asia have tumors with an EGFR driver mutation. Regardless of ethnicity, EGFR mutations are more often found in tumors of female nonsmokers. Most commonly, these patients have adenocarcinoma.
? Approved EGFR inhibitors, also known as EGFR tyrosine kinase inhibitors (TKIs)
There are currently four FDA-approved EGFR inhibitors, which are also known as epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs).
All of these are approved for first-line treatment; erlotinib (Tarceva?) and osimertinib (Tagrisso?) are also approved for additional treatments:
In addition, other EGFR inhibitors are currently being studied in clinical trials.
These drugs work by blocking the signals that activate the EGFR protein, resulting in decreased tumor growth and survival.19
Afatanib (Gilotrif?) is given as a pill once a day, 1 hour before or 2 hours after a meal.22
Erlotinib (Tarceva?) is given as a pill once a day on an empty stomach.23
Gefinitb (Iressa?) is gien as a pill once day, with or without food.24
Osimertinib (Tagrisso?) is given as a pill once a day, with or without food.25
A very common side effect of EGFR inhibitors is an acne-like rash on the scalp, face, neck, chest, and upper back. This occurs because normal skin cells have a lot of EGFR, and they must grow quickly to maintain the skin’s surface layer. Drugs that target EGFR also turn off the signal for skin cells to grow normally, and make it harder for them to retain moisture.22,23,24,25
Other common side effects of EGFR inhibitors as a group include:22,23,24,25
Serious but rare side effects that have been seen with one or more of the EGFR inhibitors are:22,23,24,25
When you start a new EGFR inhibitor, you should discuss with your doctor:
Get tips on managing treatment-related side effects.
Sometimes, treatment with an EGFR inhibitor will be the only treatment a patient receives. However, in most cases, an EGFR inhibitor is used before, together wtih, or after other treatments, which can include chemotherapy, surgery, and/or radiation therapy.
A ROS1 rearrangement is a fusion between two genes, ROS1 and another gene. As with ALK, the fusion of the two genes produces an abnormal protein that causes cancer cells to grow and spread.
About 1%-2% of patients with lung adenocarcinoma in the US and 2%-3% in East Asia have tumors with a ROS1 mutation. ROS1 fusions are more commonly found among younger patients (median age at diagnosis is 50 years), females, never-smokers, and patients with adenocarcinoma.,26,27,28,29,30
There is currently one tyrosine kinase inhibitor that has been approved for patients with metastatic NSCLC whose tumors are ROS1-positive as detected by an FDA-approved test. This is crizotinib (Xalkori?), a TKI that is also used for patients with ALK-positive tumors.15
Other ROS1 inhibitors are currently being studied in clinical trials.
Crizotinib (Xalkori?) works by blocking the signals that the abnormal ROS1 proteins send to cells to grow and divide uncontrollably. This stops the growth and spread of the cancer cells.19
Crizotinib (Xalkori?) is given as a pill 2 times a day, with or without food.15
The side effects of crizotinib (Xalkori?) on patients who are ROS1-positive are in general the same as those of patients who are ALK-positive. The most common side effects are:15,30
Most of these side effects are mild and not permanent.
When you start on crizotinib (Xalkori?), you should discuss with your doctor:
Get tips on managing treatment-related side effects.
Sometimes, treatment with crizotinib (Xalkori?) will be the only treatment a ROS1-positive patient receives. However, in most cases, crizotinib (Xalkori?) is used before, together with, or after other treatments, which can include chemotherapy, surgery, and/or radiation therapy.
Mutations in the BRAF gene occur in 1%-3% of lung adenocarcinoma patients.7 Unlike other driver mutations in lung cancer, BRAF mutations are commonly seen in lung cancer patients who are current or former smokers. The V600E mutation is the most common mutation in the BRAF gene, but other mutations (called non-V600E mutations) can also occur.,31
There is currently one FDA-approved targeted treatment for patients with metastatic NSCLC with a BRAF V600E mutation as detected by an FDA-approved test. This is a combination treatment of a BRAF tyrosine kinase inhibitor, dabrafenib (Tafinlar?), with a MEK kinase inhibitor, trametinib (Mekinist?).32,33,34 These and other targeted therapies are also being tested for patients with non-V600E BRAF mutations in clinical trials.
The combination inhibitor works by blocking the signals that the abnormal BRAF proteins send to cells to grow and divide uncontrollably. This stops the growth and spread of the cancer cells.19
Dabrafenib (Tafinlar?) is given as a pill twice daily approximately 12 hours apart and at least 1 hour before or at least 2 hours after eating. Trametinib (Mekinist?) is also given as a pill, but just once daily, at least 1 hour before or at least 2 hours after eating.33,34
Like other targeted therapies, the combination of medications used to target BRAF V600E have a unique side-effect profile.32,33,34
Some common side effects of the BRAF V600E combination treatment include:
Some rare but serious side effects of the combination inhibitor include cutaneous squamous cell carcinomas, non-cutaneous malignancies, hemorrhagic events, colitis, gastrointestinal performation, deep vein thrombosis, and pulmonary embolism.32,33,34
When you start on the combination therapy, you should discuss with your doctor:
Currently clinical trials are open for many drugs that inhibit the effect of mutations seen in NSCLC and small cell lung cancer. The targeted treatments are being studied alone, as well as in combination with other targeted agents, immunotherapy, chemotherapy, and radiation therapy. As the number of known driver mutations in lung cancer tumors increases, so does the number of drugs being developed to target them. Discuss with your doctor whether participating in a clinical trial might be a good option for you. Drugs that are currently being studied are intended to act against the following driver mutations:35
| Driver Mutation | Lung Adenocarcinoma | Squamous Cell Lung Cancer | Small Cell Lung Cancer |
| TP53 | X | X | X |
| EGFR | X | ||
| KRAS | X | ||
| MEK1 (MAP2K1) | X | X | |
| RB1 | X | X | X |
| ALK (fusion) | X | ||
| MYC | X | Rare | X |
| FGFR1 (amp) | X | X | X |
| RET | X | ||
| MET | |||
| Amplification (de novo) | X | ||
| Amplification (EGFR TKI-resistant) | X | ||
| Exon 14 skipping | X | X | |
| PTEN | X | X | X |
| PIK3CA | |||
| Mutation | X | X | |
| Amplification | X | X | X |
| BRAF | X | ||
| ROS1 | X | ||
| NTRK1 | X | ||
| HER2 | |||
| Mutation | X | ||
| Amplification | X | ||
| IGR1 | X | ||
| PARP1 | X | X | |
| Notch signaling | X |
The biggest challenge of TKIs is that a majority of patients with lung cancer who initially benefit from them eventually develop resistance. Acquired resistance is defined* as disease progression in a patient after:36,37
*According to Response Evaluation Criteria in Solid Tumors (RECIST) or World Health Organization (WHO) criteria
Cancer cells are clever enough to bypass roadblocks to their survival and often further mutate to overcome the effects of targeted drugs.
For example, the most common way adenocarcinomas become resistant to EGFR inhibitors is by mutating to a drug-resistant state that stops the drugs from working. Another way a tumor can become resistant to EGFR inhibitors is by activating a different signaling pathway in the cell to bypass the pathway that the drug uses to kill the cells.36 In a small number of cases, the adenocarcinoma may transform into small cell lung cancer.38
Similarly, lung cancers with an ALK or ROS1 rearrangement normally have good responses to ALK or ROS1 inhibitors. However, the majority of patients also eventually become resistant to the effects of the drugs. In many cases, resistance arises because of further mutations.
Doctors and scientists are working to overcome resistance in tumors and to keep the TKIs effective against cancer for longer periods of time. Their approaches include:39
If a patient’s cancer has grown after treatment with targeted therapy, a decision needs to be made about the next treatment option. Your doctor may recommend that a biopsy be done of one of the tumors that is growing to determine whether there is a new mutation. For example, for EGFR patients, if the (EGFR) T790M mutation is present (it is found in about two-thirds of patients who have this biopsy), your doctor may recommend the next-generation EGFR inhibitor, osimertinib (Tagrisso?) or a clinical trial.39
In addition to osimertinib (Tagrisso?) for EGFR patients, several other next-generation inhibitors have already been approved, including ceritinib (Zykadia?, alectinib (Alecensa?), and brigatinib (Alunbrig?) for ALK-positive NSCLC patients.16,17,18 Scientists are researching approaches to overcome resistance to crizotinib (Xalkori?) in ROS1-positive lung cancer and learning about acquired resistance in BRAF-positive lung cancers.
Targeted therapies are aimed at specific pathways that tumor cells use to thrive, blocking them in the same way that blocking a car's fuel line would keep it from running properly. The advantage of such precise treatments is that they can target the root cause of why a tumor is growing, which may make them more effective.19
If you are considering participating in a clinical trial, start by asking your healthcare team whether there is one that might be a good match for you in your geographic area. In addition, there are several resources to help you find one that may be a good match.
Resources to help you navigate your clinical trials search:
In addition, if you are interested in a specific drug or other treatment that is being developed, you can often find information about studies for that drug on the website of the company developing it.
Learn more about clinical trials here.
To find out whether targeted therapy is appropriate for a person who has been diagnosed with lung cancer, that person's tumor tissue will be tested for the presence of driver mutations. Patients who have a mutation that a specific FDA-approved targeted therapy targets are candidates for that treatment. The process of testing for a mutation in a tumor is called biomarker testing (also know as mutation, genomic, or molecular testing).
Note: At this time, tissue biopsies are the only way to confirm a diagnosis of lung cancer and to detect driver mutations. However, liquid biopsies, which make use of blood, can sometimes be used to look for resistance mutations like (EGFR) T790M and are being studied in other contexts.40,41

Biomarker testing should be an ongoing part of the discussion with our doctors. Any decision to test for biomarkers should be made together, and will depend on a number of factors, including your type and stage of lung cancer, your current treatment plan, your overall health, and your preferences.
Note that biomarker testing may also be used to determine whether an immunotherapy drug is appropriate. In this section, however, we are only discussing biomarker testing that will help doctors determine whether a targeted therapy is an appropriate treatment.
Once the decision has been made to do biomarker testing, a surgeon will remove either the entire tumor (surgery) of part of a tumor (biopsy). Be sure to confirm with your doctor that adequate tissue will be gathered so that all necessary biomarker tests can be performed.
There are a number of tissue collection techniques, including bronchoscopy, endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), transthoracic needle biopsy, thoracoscopy, and thoracentesis.40 Tumor tissue is then sent to a laboratory that can test it for driver mutations. Test results are generally available within 10 to 14 days. Biomarker testing can be done on both primary tumors and metastatic tumors.41,42
Multiplex testing—testing for multiple gene mutations at the same time from the same sample of tumor tissue—is currently used in some laboratories. This allows more testing to be done on a small tumor sample. An example of multiplex testing is next-generation sequencing, or NGS.43
Again, the decision to have your tumor tested and when depends on a number of factors. Below are common recommendations for biomarker tesing for driver mutations.40,41,42,44
| Type of Lung Cancer | Stage of Lung Cancer | Recommendations for Biomarker Testing for Driver Mutations |
| Adenocarcinoma | Stage I, II, or III | Testing for the ALK, EGFR, KRAS, ROS1, and BRAF V600E mutations at the time of diagnosis |
| Stage IV or adenocarcinoma that has recurred or progressed after an initial diagnosis of stage I, II, or III in patients who were not previously tested | Tumors should be tested for ALK, EGFR, KRAS, ROS1, and BRAF V600E mutations at the time of diagnosis. Testing for other biomarkers may be helpful in deciding eligibility for clinical trials While there is no approved targeted therapy for the KRAS driver mutations, testing for it can be informative because cancers with KRAS mutations are very unlikely to have other driver mutations. Targeted therapies for KRAS-positive cancers are being developed in clinical trials. Additionally, KRAS mutations can be associated with resistance to EGFR targeted therapy | |
| Squamous cell lung cancer | Stage I, II, or III | Currently, biomarker testing is performed only for clinical trials |
| Stage IV | If your doctors suspect that the tumor may have adenocarcinoma cells (this type of lung cancer is referred to as mixed lung cancer with an adenocarcinoma component), testing for ALK and EGFR mutarions is recommended. Otherwise, biomarker testing is currently only performed for clinical trials. | |
| Small cell lung cancer | All stages | Currently, biomarker testing is performed only for clinical trials |
Testing to identify other possible mutations in the tumor may help you find clinical trials. These trials are testing new treatments for mutations in other types of lung cancer. Therefore, you should consider biomarker testing for other mutations if tests for ALK, EGFR, ROS1, or BRAF mutations are negative.
Questions to ask your healthcare team if you are considering biomarker testing:
Before getting biomarker testing:
聯(lián)系客服

微信登錄中...
請(qǐng)勿關(guān)閉此頁面
